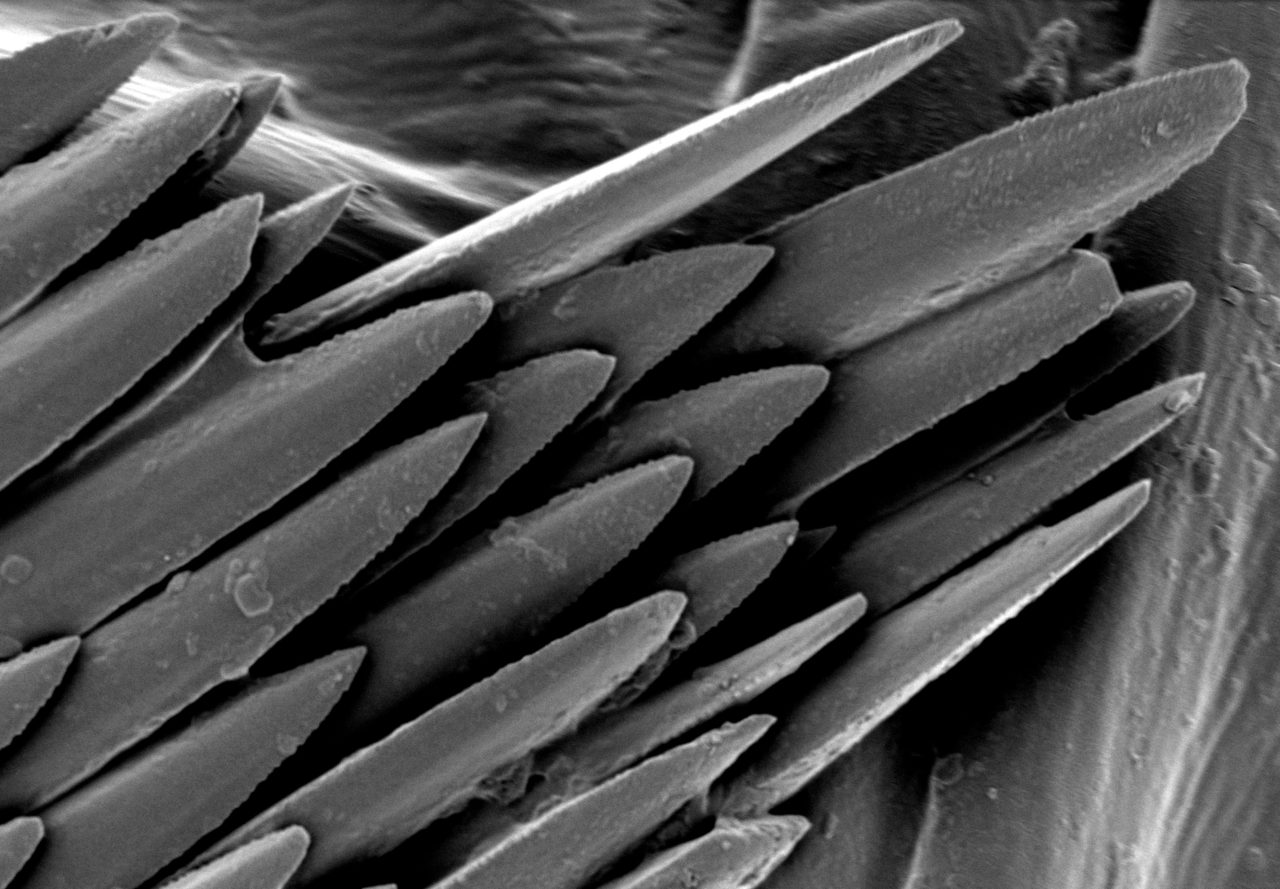
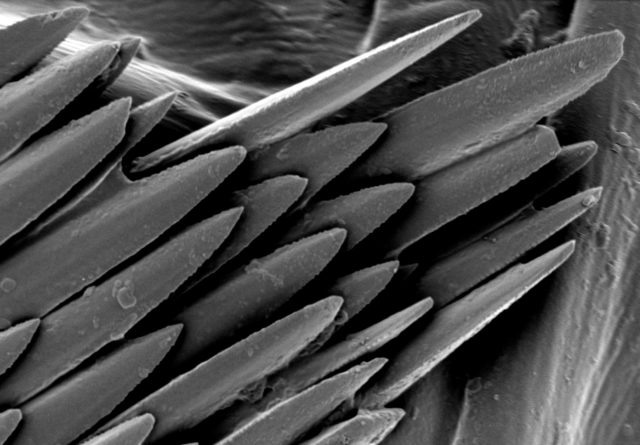
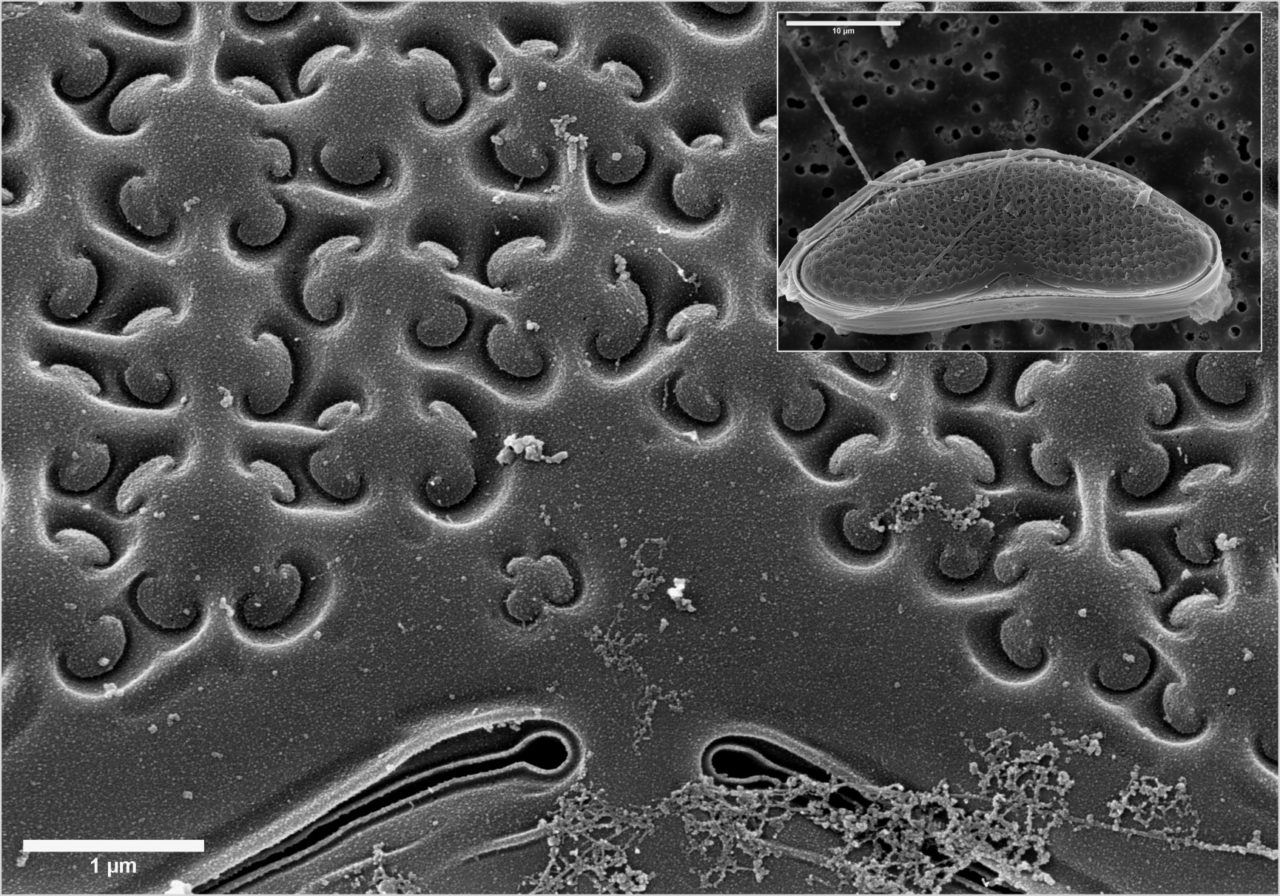
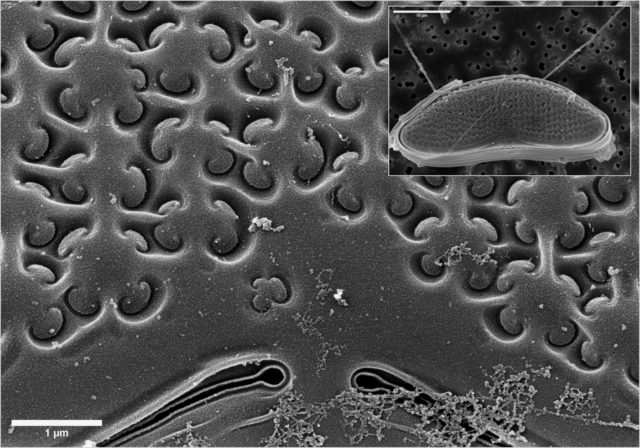
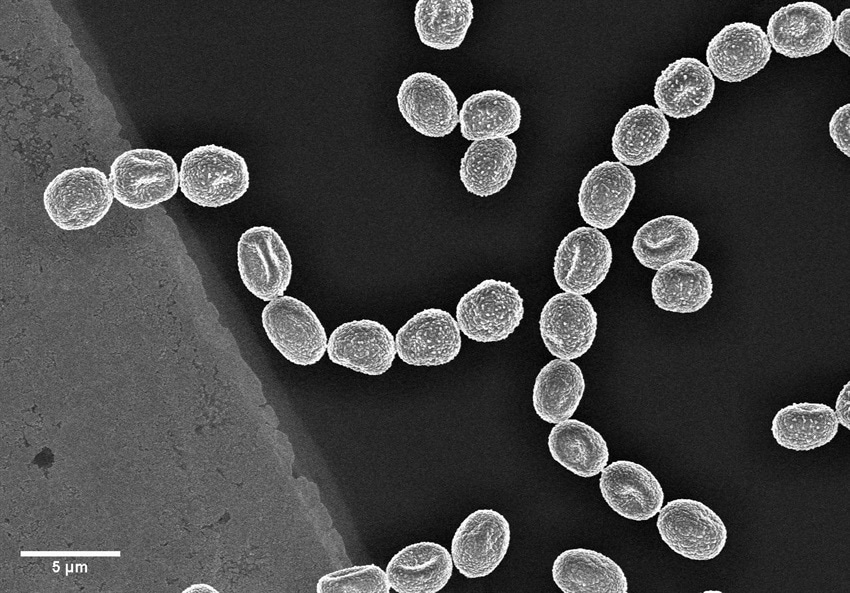
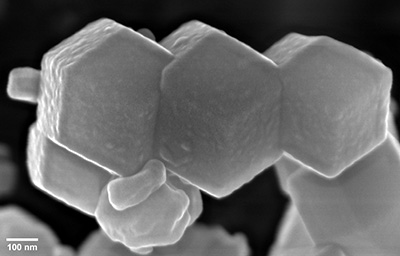
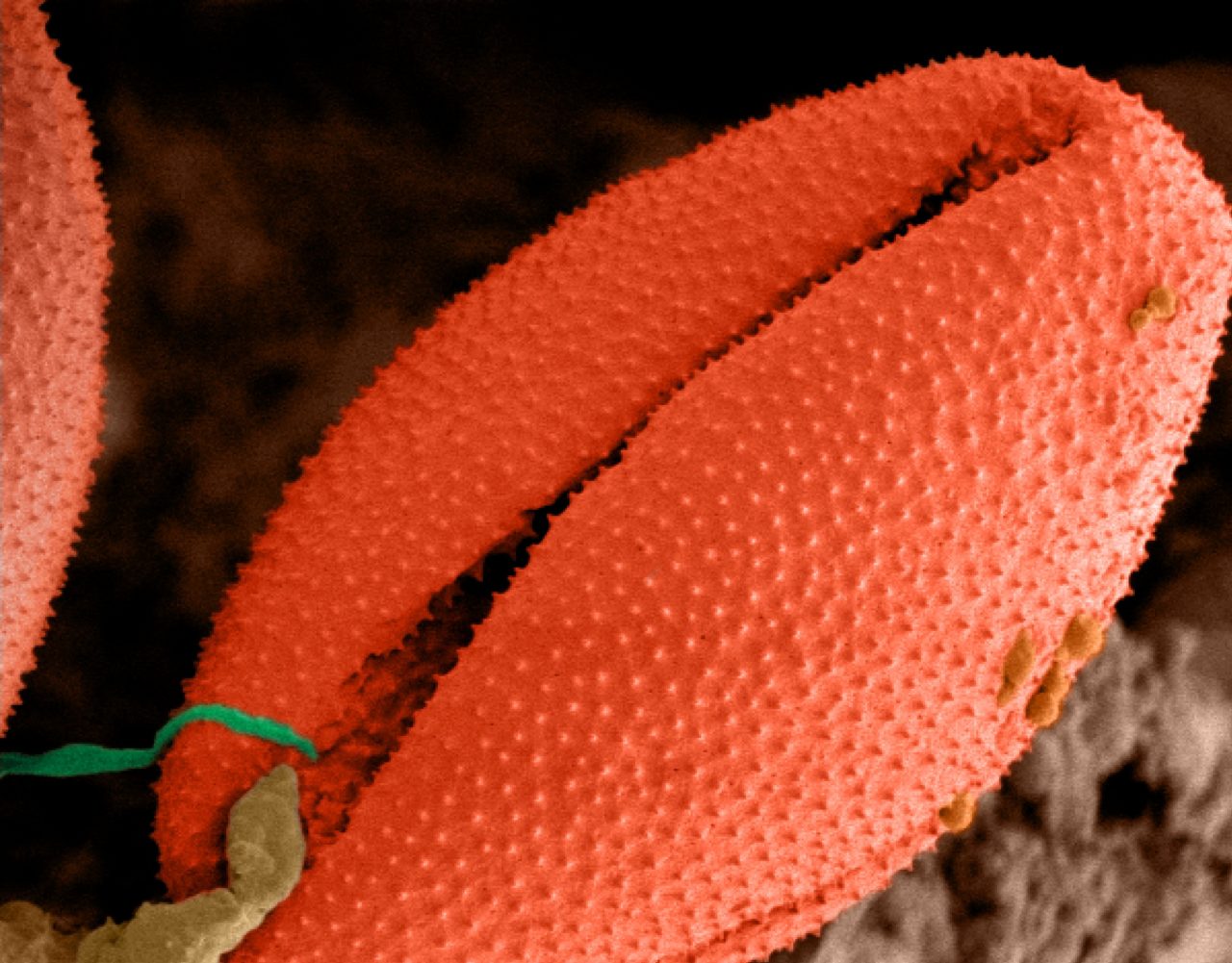
Electron microscopes use electrons rather than visible light to investigate and image samples, thus providing orders-of-magnitude enhancements on the magnification and resolution of traditional optical microscopes.
However, electron microscopes are much more complicated than optical microscopes, and the conditions that samples are subjected to are very difficult to work with.
SEM and TEM both depend on the transfer of electrons between a sample and the microscope itself, and thus imaging is performed under high vacuum. Moreover, samples must be conductive to enable electron transfer. This poses a difficulty for some sample types.
Although it is comparatively easy to use SEM or TEM to image solid metallic samples, for instance, imaging soft, hydrated and/or non-conductive samples — for example, biological tissues — can be challenging.
When left untreated, such samples lead to several issues. Moisture and gas from the sample easily evaporate inside the vacuum environment of an electron microscope sample chamber, thereby contaminating the microscope and damaging the sample.
Moreover, upon subjecting non-conductive samples to the incident electron beam within an electron microscope, the electrons do not conduct through the sample, making them accumulate in one place. This “charging” results in several imaging artifacts and can even make it impossible to image samples.
It is possible to solve these issues by the careful preparation of samples, specifically by sample coating. Using a layer of conductive material (carbon or metal) to coat samples has several purposes. There are two key reasons for coating: to make the sample conductive, which avoids “charging” effects, and to encapsulate samples to eliminate off-gassing or evaporation.1
Specifically, metallic coatings can facilitate better thermal conductivity, safeguarding the sample from heat damage from the incident electron beam. They can even localize the signal to the actual surface of the sample, thus enhancing the signal-to-noise ratio and secondary electron emission.
Carbon coatings offer certain distinct advantages. Carbon coatings for electron microscopy are amorphous, conductive layers transparent to electrons. This implies that carbon coatings are particularly valuable for making non-conductive samples amenable to energy-dispersive x-ray spectroscopy (EDS).2
Achieving High-Quality Carbon Coatings
For SEM and TEM applications, metallic coatings such as tungsten and gold can be done through sputtering. However, the same is not true of carbon. Although carbon can be sputter-coated, the resulting coatings exhibit high hydrogen concentrations, which makes carbon sputtering unsuitable for electron microscopy applications.
Alternatively, high-quality carbon coatings can be performed through thermal evaporation of carbon in vacuum. Two similar techniques can be used to achieve this — using carbon fiber or using a carbon rod.
In the carbon rod coating method, two carbon rods with a sharpened contact point between them are used. This is also called the Brandley method.
The process involves passing current between the two rods, ensuring very high current density at the sharpened contact point, which leads to very high levels of resistive heating. This causes the evaporation of carbon from the surface. This can be achieved with a ramped current or a pulsed current.
In the carbon fiber technique, a carbon fiber is mounted between two clamps, and a pulsed current is passed along it. This leads the carbon to evaporate from the surface of the fiber.
Both techniques exhibit unique differences in quality. The carbon fiber technique facilitates certain control over coating thickness by tweaking the number of current pulses and a pulse length. This makes it appropriate for TEM grid applications and analytical SEM applications like EDS and electron backscatter diffraction (EBSD). Yet, pulsed carbon fiber coatings essentially contain higher levels of debris.
Carbon rod coatings are “cleaner” and of better quality. Carbon rod coatings made in high vacuum with ramped current offer the maximum quality coatings, suitable for high-resolution TEM applications and crucial SEM applications.
In its pulsed version, the technique can be employed to achieve thicker coatings for SEM, particularly for wavelength-dispersive X-ray spectroscopy (WDS) and EBSD. In such applications, it is important to select carbon rods with the maximum purity to achieve the maximum possible coating quality.
Carbon Coating Solutions from Quorum Technologies
Quorum Technologies has come up with the new Q Plus Series that offers an all-in-one solution to attain high-quality carbon coatings for all electron microscopy applications.
With the ability to coat both carbon fiber/cord and carbon rod, the carbon evaporators employ easy-change inserts to allow users to easily switch between the two modes.
The new Q150V Plus from Quorum Technologies offers the highest vacuum of 1 x 10−6 bar for superior results. The lower background pressure implies oxygen, nitrogen and water vapor are eliminated from the coating chamber, restricting chemical reactions while executing the coating process. This leads to impurities or defects. Lower scattering also implies amorphous carbon films of higher purity and high density.
References
- Goldstein, J. I. et al. Coating Techniques for SEM and Microanalysis. in Scanning Electron Microscopy and X-Ray Microanalysis: A Text for Biologist, Materials Scientist, and Geologists (eds. Goldstein, J. I. et al.) 461–494 (Springer US, 1981). doi:10.1007/978-1-4613-3273-2_10.
- Heu, R., Shahbazmohamadi, S., Yorston, J. & Capeder, P. Target Material Selection for Sputter Coating of SEM Samples. Microscopy Today 27, 32–36 (2019).
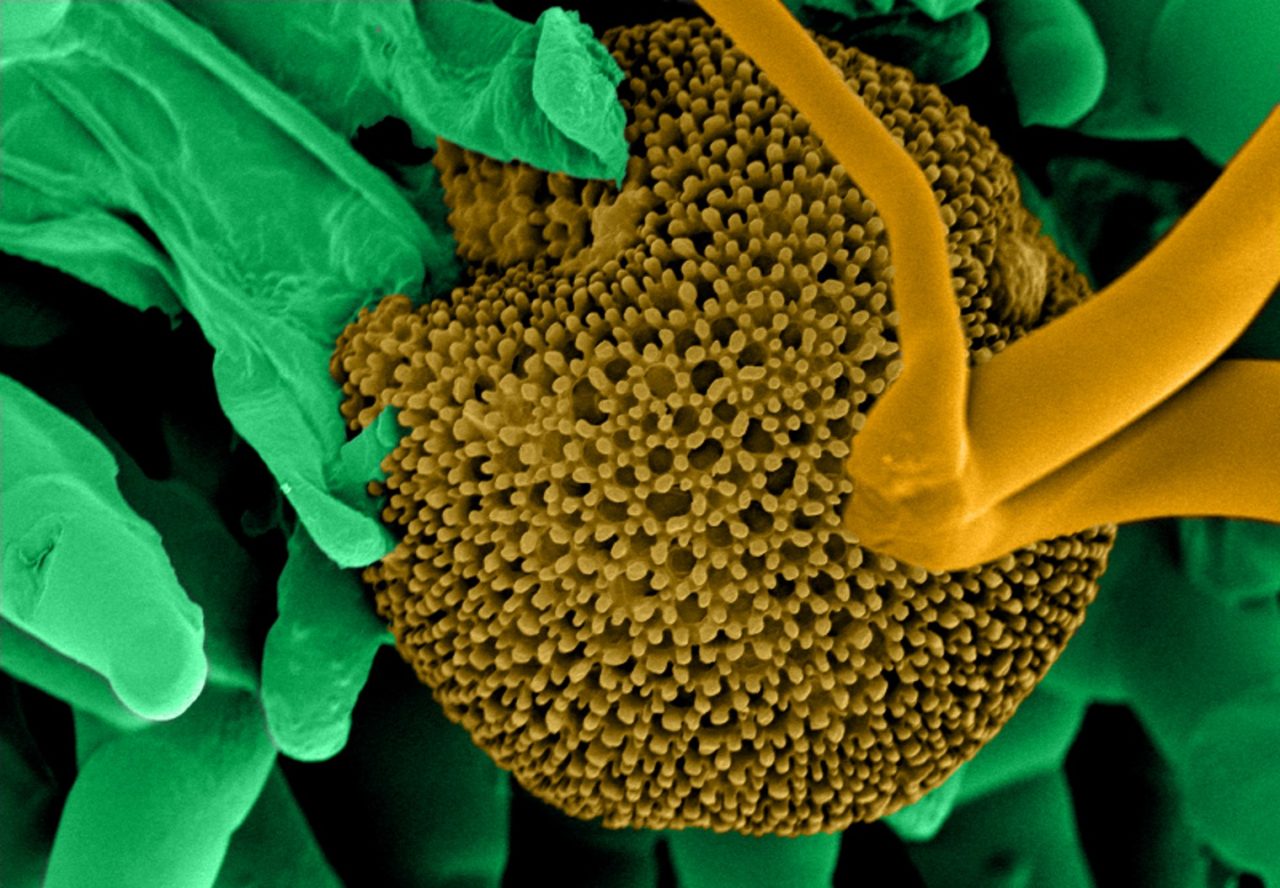
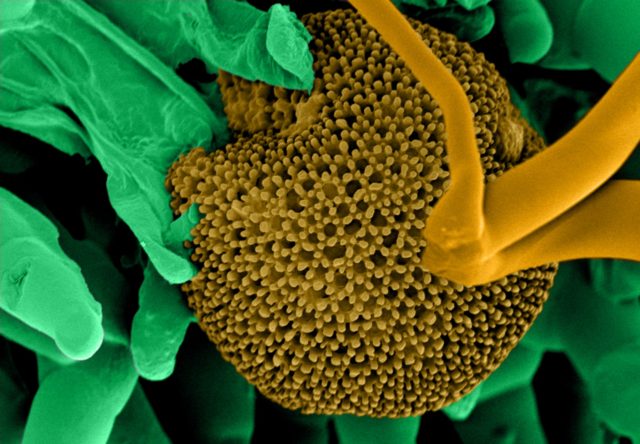
- Image shown – Fungi spores on a TEM grid (Cu, 300 mesh with 5nm carbon film produced with Q150VES Plus coater). Image Credit: Quorum Technologies


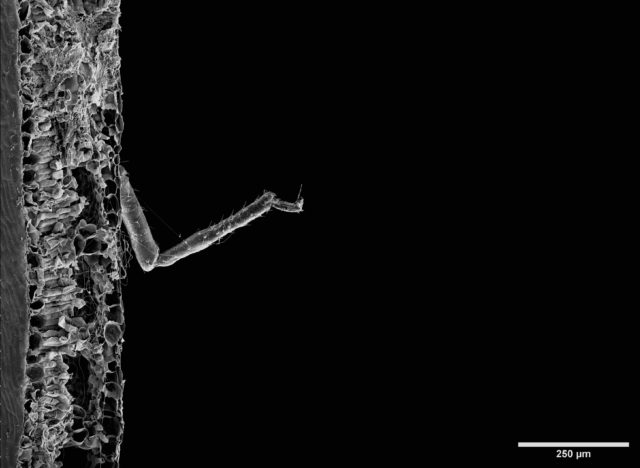
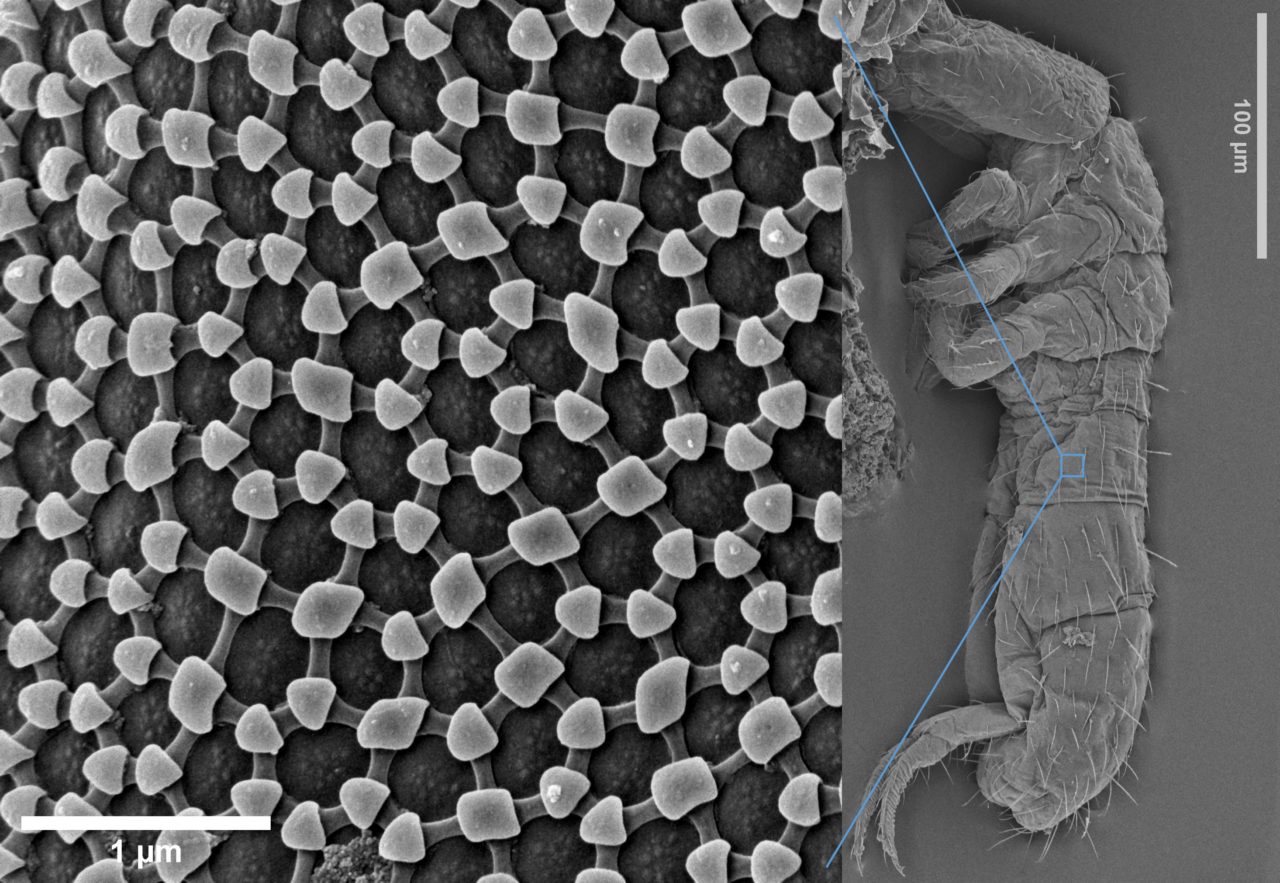
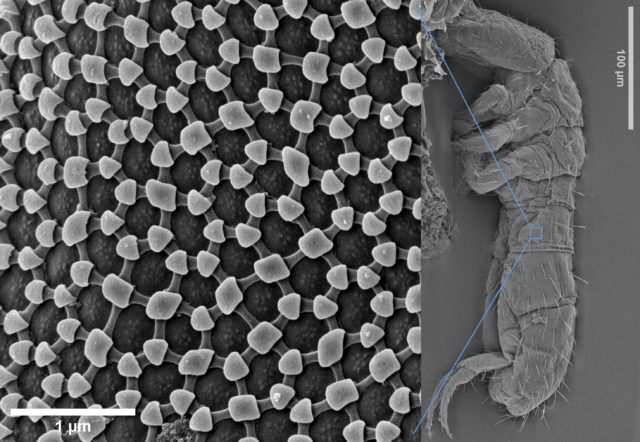
 distinctive structural features, i.e., variation in size, shape, number of apertures and surface texture, which also makes it possible to identify and classify a plant at family and genus level, and often even species level.
distinctive structural features, i.e., variation in size, shape, number of apertures and surface texture, which also makes it possible to identify and classify a plant at family and genus level, and often even species level.